Abstract
Background: AML is a biologically and clinically heterogenous disease that affects a diverse patient (pt) population of both sexes and all ages. Despite recognition of differences in frequencies with which cytogenetic and molecular alterations occur in male (M) and female (F) AML pts, no large, global study has assessed potential associations between sex and prognostic impact of recurrent genetic alterations.
Aims: To (1) establish the frequencies of pretreatment cytogenetic and mutational features in M and F pts, including patterns of co-existing mutations, (2) assess the impact of established molecular prognosticators in pts of each sex, (3) delineate the impact of sex-associated genetic alterations on survival outcomes, and (4) characterize individual sex-specific markers, including gene-expression profiles and differentially expressed splicing patterns, to provide potential biologic insights into molecular and biologic contributors to observed sex differences.
Methods: We compared molecular features and clinical outcomes in 1726 pts (F, n=749, M, n=977), similarly treated on frontline Alliance for Clinical Trials in Oncology protocols. A second cohort treated on frontline protocols of the German AML Cooperative Group (AMLCG; n=954 pts, F, n=465, M, n=489) was used for validation of molecular features. The mutational status of 43 protein-coding genes was determined by both groups centrally using targeted NGS platforms.
Results: F pts more often had cytogenetically normal AML and less often a complex karyotype. A higher percentage of F pts was categorized in the 2017 European LeukemiaNet (ELN) Favorable-risk group (44% vs 40%) with a lower percentage of F pts in the ELN Adverse-risk group (28% vs 40%). Mutational analysis revealed several molecular differences between F and M pts. F pts harbored more FLT3-ITD and mutations in DNMT3A, NPM1, and WT1 genes and fewer mutations in ASXL1, KIT, RUNX1, SRSF2 and U2AF1 genes. Differences in ELN risk group allocations and all sex-associated mutation patterns, except frequency differences of KIT and WT1 mutations, were validated in the AMLCG cohort.
Patterns of co-occurring mutations varied between sexes, with NRAS/BCOR, U2AF1/CBL, NOTCH1/BCORL1 and SF1/SRSF2 demonstrating a strong positive association in F pts and ASXL1/TET2, RUNX1/PHF6, RUNX1/U2AF1 and RUNX1/STAG2 strongly associated in M pts.
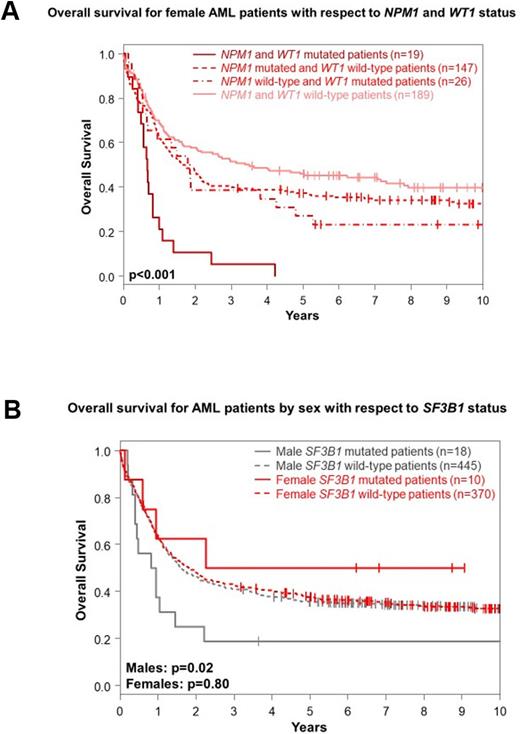
Outcomes of F and M Alliance pts did not differ significantly in any tested endpoint, including complete response and early death rates, disease-free or overall survival (OS). In the final multivariable models for OS, presence of FLT3-ITD, CBF-AML, complex karyotypes and TP53 mutations impacted both M and F pts concordantly. However, RUNX1 and WT1 mutations only associated with shorter OS in F pts (WT1, 5-year rates, 16% vs 41%, P<0.001). Inferior survival of WT1-mutated F pts seemed to be driven by co-existence of NPM1 and WT1 mutations (Fig A). In contrast, NPM1 and CEBPAdm were associated with longer and SF3B1 mutations with shorter OS in M pts (5-year rates, 17% vs 36%, P=0.02, Fig. B). Though CEBPAdm conferred a longer OS in univariable analysis also in F pts, neither NPM1 nor SF3B1 mutations associated with OS in F pts.
Differences in sex-specific alternative splicing events in spliceosome genes were present in M and F pts with distinct lineage enrichments among M and F pts with SF3B1 mutations. In gene set enrichment analyses, M pts with SF3B1 induced genes were associated with integrin cell-surface receptor linked signaling pathways and involved dominant megakaryocytic/erythroid progenitors. Conversely, F pts with SF3B1 mutations were enriched in hematopoietic stem cells, common lymphoid precursors and eosinophil/mast cell programs.
Conclusions: Our study confirmed sex-associated differences in frequencies of somatic mutations in AML, including a predominance of common AML mutations in NPM1, DNMT3A and FLT3-ITD in F pts, and a higher incidence of spliceosome mutations in M pts. We also showed adverse prognostic impacts of WT1 mutations in F pts and SF3B1 mutations in M pts and revealed sex-associated differences in co-mutational patterns. To our knowledge, this is the first large study to analyze sex-associated markers on a global scale and correlate them with pts' outcomes.
Support: U10CA180821, U24CA196171; https://acknowledgments.alliancefound.org. Clinicaltrials.gov Id: NCT00048958, NCT00899223, NCT00900224
Disclosures
Larkin:Gilead: Membership on an entity's Board of Directors or advisory committees. Blachly:MingSight Pharmaceuticals: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; INNATE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Walker:Karyopharm Therapeutics: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Krug:AbbVie: Honoraria; , Sanofi: Honoraria; BMS: Honoraria; , Leo Pharma,: Honoraria. Subklewe:Roche: Consultancy, Research Funding; Takeda: Other: Travel support; Novartis: Consultancy, Speakers Bureau; Morphosys: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Pfizer: Consultancy; Janssen: Consultancy, Speakers Bureau; Seagen: Research Funding; Miltenyi Biotech: Research Funding; Gilead: Consultancy, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau. Powell:Ambit Biosciences: Research Funding; Genentech: Research Funding; Hoffman LaRoche: Research Funding; Jazz Pharmaceuticals: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Rafael Pharmaceuticals: Consultancy, Research Funding. Moore:Pharmacyclics: Speakers Bureau. Stone:Innate: Consultancy; GSK: Consultancy; Syntrix: Consultancy; Janssen: Consultancy; Astellas: Consultancy; BMS: Consultancy; Gemoab: Consultancy; Boston Pharmaceuticals: Consultancy; BerGenBio: Consultancy; Syros: Consultancy; Epizyme: Consultancy; Foghorn Therapeutics: Consultancy; Jazz: Consultancy; Kura Oncology: Consultancy; Syndax: Consultancy; Elevate Bio: Consultancy; OncoNova: Consultancy; Apteva: Consultancy; Actinium: Consultancy; Aprea: Consultancy; Arog: Consultancy, Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Research Funding; Takeda: Consultancy. Stock:Newave Pharmaceuticals: Consultancy; MorphoSys: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Pluristem: Consultancy, Honoraria; Servier: Honoraria; Syndax: Consultancy, Honoraria; Kura Oncology: Honoraria; Kite: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Agios: Honoraria. Metzeler:Daiichi Sankyo: Honoraria; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy, Honoraria, Research Funding; Curis: Research Funding; Astellas: Honoraria; AbbVie: Honoraria. Byrd:AstraZeneca: Consultancy; TG Therapeutics: Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy; Syndax: Consultancy; Kura Oncology, Inc: Consultancy; Pharmacyclics LLC: Honoraria, Research Funding; Xencor, Inc: Research Funding; Vincerx Pharma: Current equity holder in publicly-traded company; Novartis: Consultancy, Honoraria. Mims:Daiichi Sankyo: Other: Data Safety and Monitoring Board; Zentalis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Other: Data Safety and Monitoring Board; Servier: Membership on an entity's Board of Directors or advisory committees; Ryvu: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Eisfeld:Karyopharm Therapeutics: Other: Spouse is current company employee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal